Heat treating brings out the magic in steel. Steels with a carbon content higher than about .3% can be hardened through heat treating. Try this out at home with a piece of music wire, heat the wire to red hot then quickly submerge it in water. The wire will be as brittle as glass, try the same thing with a paperclip and it will stay ductile. The reason is because a paperclip is low carbon, its molecular structure doesn't change enough during heating to become hardened.
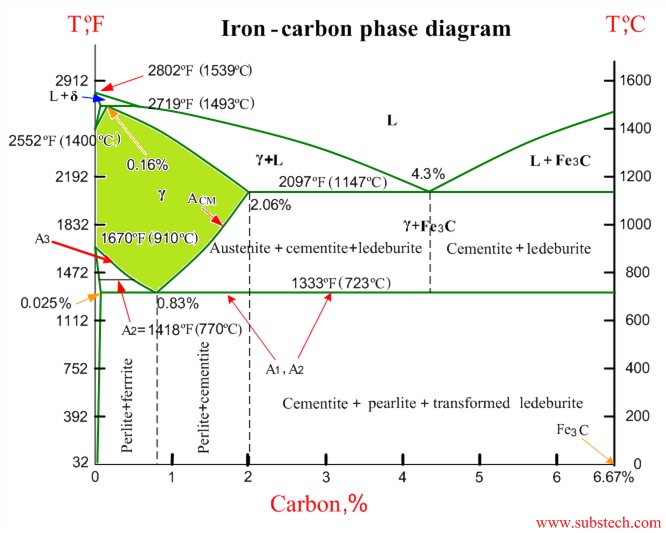
- SteelPhaseDiagram.jpg (83.52 KiB) Viewed 2920 times
The iron carbon phase diagram shows how the percentage of carbon affects the formation of austenite, the area in green is of most interest. Heat treating takes place between 1400-1600 degrees F, at these temperatures the molecular structure of the steel changes where the carbon dissolves into the steel, much like salt dissolves into hot water, by quenching the steel from an elevated the molecular structure gets trapped in the steel and provides desirable properties.
Alloys such as tungsten, vanadium and chromium encourage formation of carbides. These carbides are microstructures with beneficial hardness and wear resistance.
In carbide structure, two carbon atoms are attached to each other with the three covalent bonds. From these three covalent bonds, two bonds are pi-bonds. These pi-bonds are formed by the lateral overlapping of the p-orbitals. Another bond is the sigma bond, which is formed by the head-on overlapping of the s-orbitals. The hybridization exhibited by the carbon in the carbide structure is sp. Each carbon carries one lone pair on it.
More carbide is not always a good thing, large carbide grains make sharpening difficult and edge stability may become compromised.
The current "best" steel in the knife industry is CPM Magnacut by Crucible steel. I'll refer to this datasheet throughout the net:
https://www.alphaknifesupply.com/Pictur ... Cut-DS.pdf
CPM MagnaCut is a unique powder metallurgy stainless tool steel
with a design which eliminates chromium carbide in the heat
treated microstructure. An excellent combination of toughness and
wear resistance is achieved by having only small, high hardness,
vanadium and niobium carbides, giving CPM MagnaCut
properties similar to non-stainless steel CPM 4V.
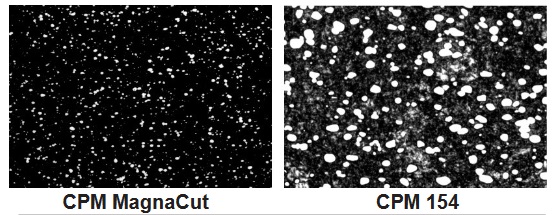
- Microstructure.jpg (75.11 KiB) Viewed 2920 times
The bright white dots in these two images represent carbides. Chromium carbides tend to be larger (microscopically speaking) while very hard and wear resistant, large carbides can result in chipping at the edge as entire carbide grains rip out of the blade under stress.
Normally the grain in metal is not visible without magnification, but zinc readily forms large grains, if you've seen a galvanized fence post or street lamp you've probably noticed the spangled appearance, the splotchy shapes are grains in the metal coating.

- Galvanized Pipe.jpg (129.38 KiB) Viewed 2920 times
The molecules cluster together in specific orientations until an impurity disrupts the organization of molecules, then they will spontaneously cluster in another orientation until an impurity upsets the organization again. The grain boundaries are an area of weakness and unpredictability. By making the grain boundaries as small as possible the metal becomes more stable.